Treatment choice is beneficial for people living with rare diseases (RDs) and competition can drive important economic advantages for payers.
For drug manufacturers, however, competition in RDs poses a unique set of challenges. With an increasing number of RDs served by multiple approved therapies, we are seeing the emergence of competitive arenas and a lower return on investment for orphan drug incentives, impacting where and how industry must focus their efforts to meet payer evidence expectations and effectively position RD assets in an evolving environment.
16 Rare diseases with >2 treatments in 2015
» 101 Rare diseases with >2 treatments in 2025
7 Rare diseases with >3 treatments in 2015
» 54 Rare diseases with >3 treatments in 2025
Critical success factors and select considerations for manufacturers in competitive RDs
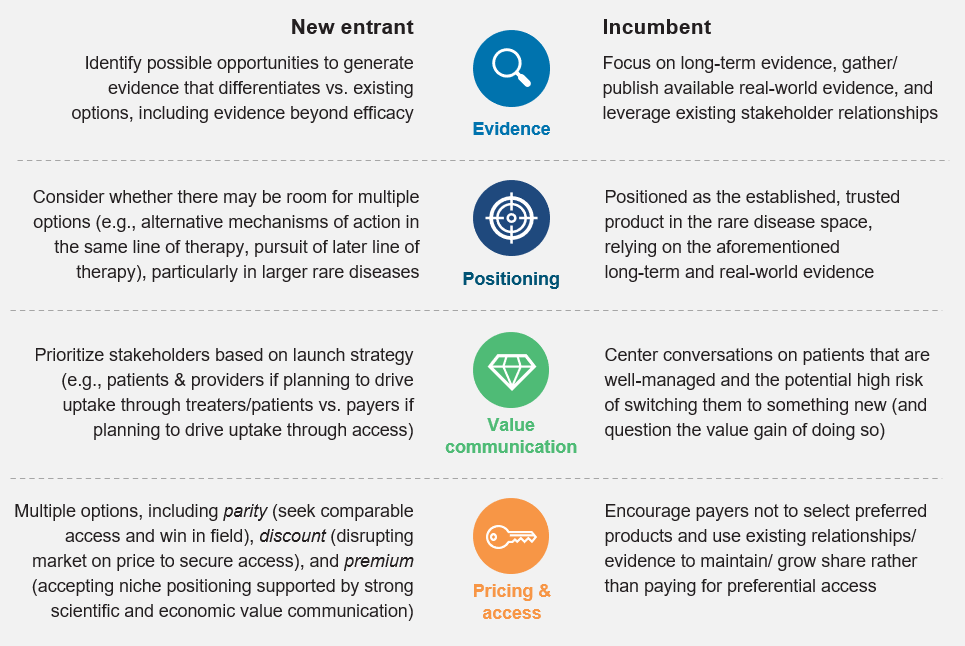
Increasingly, RD manufacturers will need to navigate the transition to competitive categories, creating challenges for both incumbents and new entrants to maintain and preserve value. The industry must ensure cross-functional preparedness and an agile strategy to succeed in these competitive environments.
Sources: FDA Orphan Drug Designations and Approvals Database




